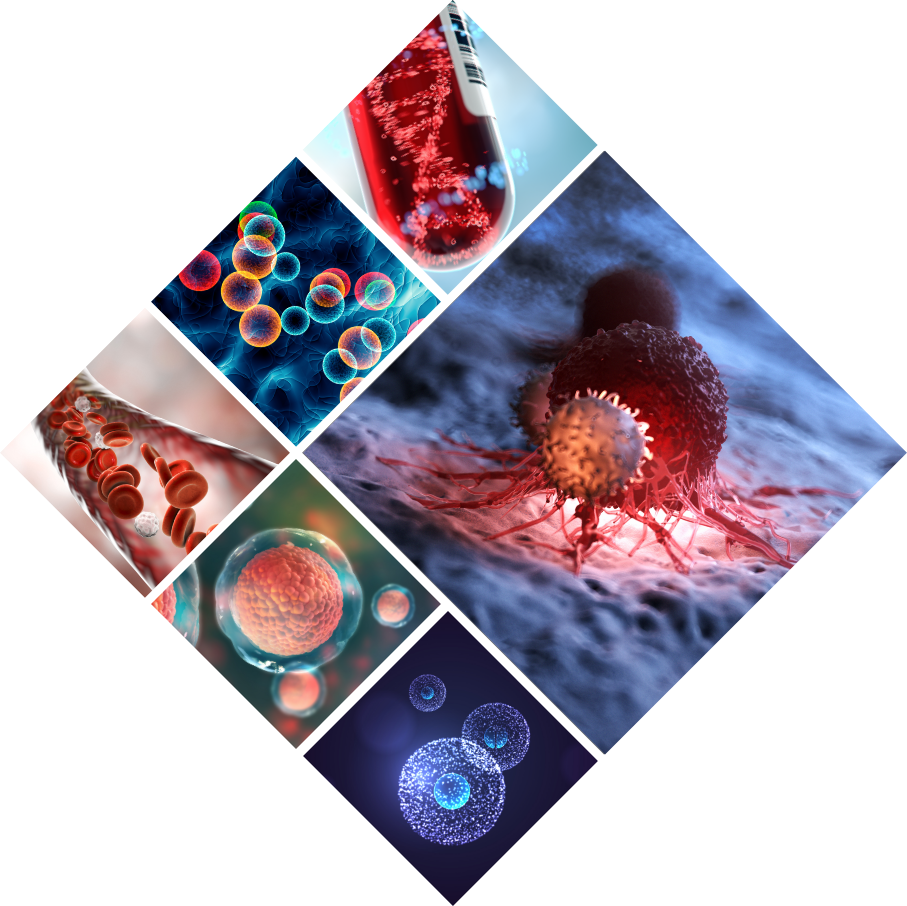
In the year 2000, Dr. Ilan Morad had an idea as to how to create what can be described as a functional Phage Display. With this idea he applied for the ITEK incubator in the Weizmann Science Park. Six years later, AEBi was able to prove that the novel technology, titled ‘SoAP’, really works.
Within the Phage Display family, and unlike other members of this family of technologies, AEBi’s platform provides functional leads to very difficult targets (functional leads – agonist, antagonist, inhibitor, etc.), not merely those that best bind with the target.


In the year 2000, Dr. Ilan Morad had an idea as to how to create what can be described as a functional Phage Display. With this idea he applied for the ITEK incubator in the Weizmann Science Park in Israel. Six years later, AEBi was able to prove that the novel technology, titled 'SoAP' (IP protected), really works. The platform is very flexible, exploiting combinatorial biology to discover new and better lead compounds to disease targets. The major advantage is that it can be used not only to find binders to known targets, but to select the best functional molecules among them. AEBi has been managed since then based on the idea that the ability to manipulate and design peptides has vastly superior advantages to many, existing and trajectory technologies of drug development. This idea is valid today as well.
Focusing on the science & technology on the critical path to proof of concept
Don't water down the solution in order to generate Investor\Pharma\Media traction
Research & Development of a comprehensive fundamental idea
There is no clinical tryout at the moment of MuTaTo in Israel or outside of Israel.
Our team are research scientists (Ph.D.) and are not attending Dr.
We are working now for the 20th year entering our 21th year of R&D effort (in June 2022).
We don’t have a Clinique and we can not advise nor refer to any specific case.
We initially intend to target: lung, colon, head & neck cancers.
The treatment, in any case, has to be individually adapted to the patient.
We are a small team and have an influx of e-mail.
We try to answer at least initial emails but can not promise it.
This website may contain certain statements, estimations, projections, assessments and other information with respect to the anticipated future performance of the Company and/or its market and involve risks and uncertainties. Actual results or future business decisions of the Company may differ materially from any expectations, projections or predictions made or implicated in statements made in this website. While the Company believes that the information contained herein is accurate, it expressly disclaims any and all liability for representations or warranties, expressed or implied, contained in, or for omissions from, this website or any other communication transmitted or made available herein. Any prospective website user acknowledges his/her responsibility to perform a due diligence review prior to consummating a transaction with the Company. Included in this website are certain declarations by the Company with respect to its anticipated future performance. These declarations are based on estimates and assumptions by the Company that are subject to economic and competitive uncertainties beyond the control of the Company. The Company does not provide representation or warranty with regard to the declarations and there can be no assurance that the future results will be realized or that actual results will not be significantly different from those projected. The Company is not obligated to update or revise any future declarations. The Company and its respective affiliates, directors, officers, employees, consultants and representatives expressly disclaim any and all liability relating to or resulting from the utilization of any information contented in this website. The Company does not have any obligation to provide with access to additional information.